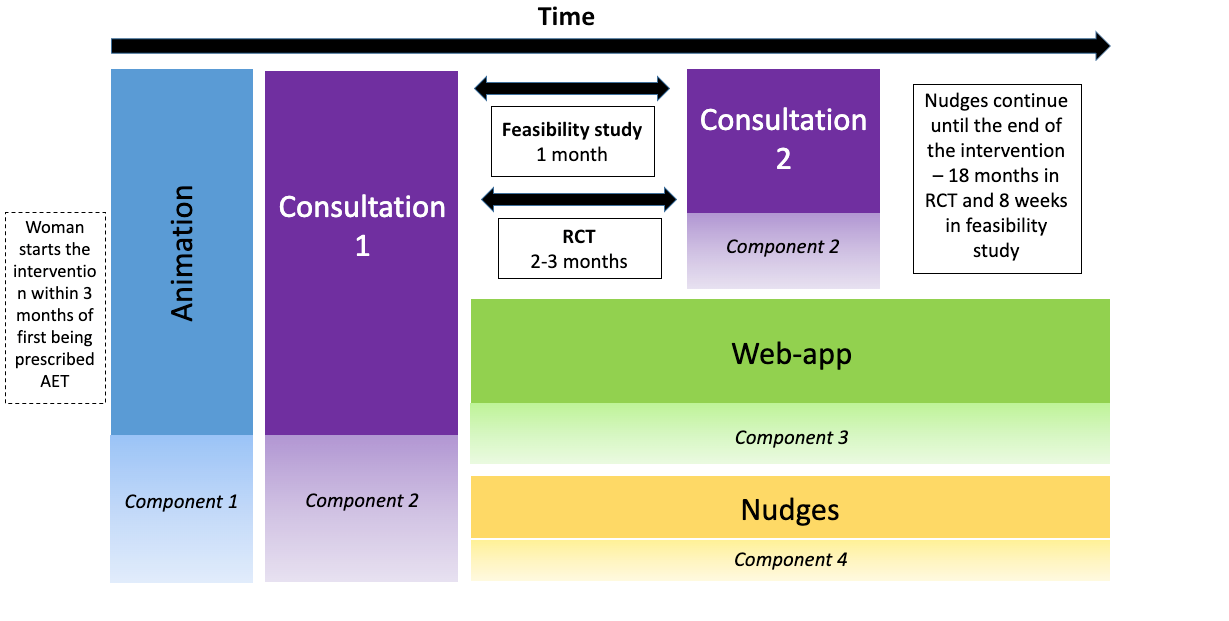
The SWEET programme has developed and completed feasibility testing of the HT&Me support package. A randomised controlled trial is now underway to trial investigate the effectiveness of HT&Me in reducing poor adherence to hormone therapy and improving quality of life in women with early breast cancer. The HT&Me support package includes:
- A short animation video explaining how hormone therapy works, why it is important to take daily, and advice for managing side-effects
- Two appointments with HT&Me Study Nurses/Practitioners
- Access to the interactive HT&Me website, accessible on a desktop computer, laptop computer, tablet or mobile phone
- Regular text or email messages reminding of the importance of hormone therapy and signposting back to the HT&Me website
Sites are open for trial recruitment across the UK. We are seeking to recruit a total of 1460 women with early breast cancer, who will be randomised to receive either:
i) the HT&Me support package alongside their usual care
or
ii) usual care alone
Women will be asked to complete a questionnaire when they sign up to the study (baseline), and then again 6,12 and 18-months later. These questionnaires will measure adherence to hormone therapy, quality of life, and other factors that might impact the effectiveness of the HT&Me support package.
To read about our earlier intervention development work and feasibility study click here.
We are also conducting a cost-effectiveness evaluation and developing a scale-up implementation strategy- to read about these click here.
The programme includes five workstreams, as shown below.
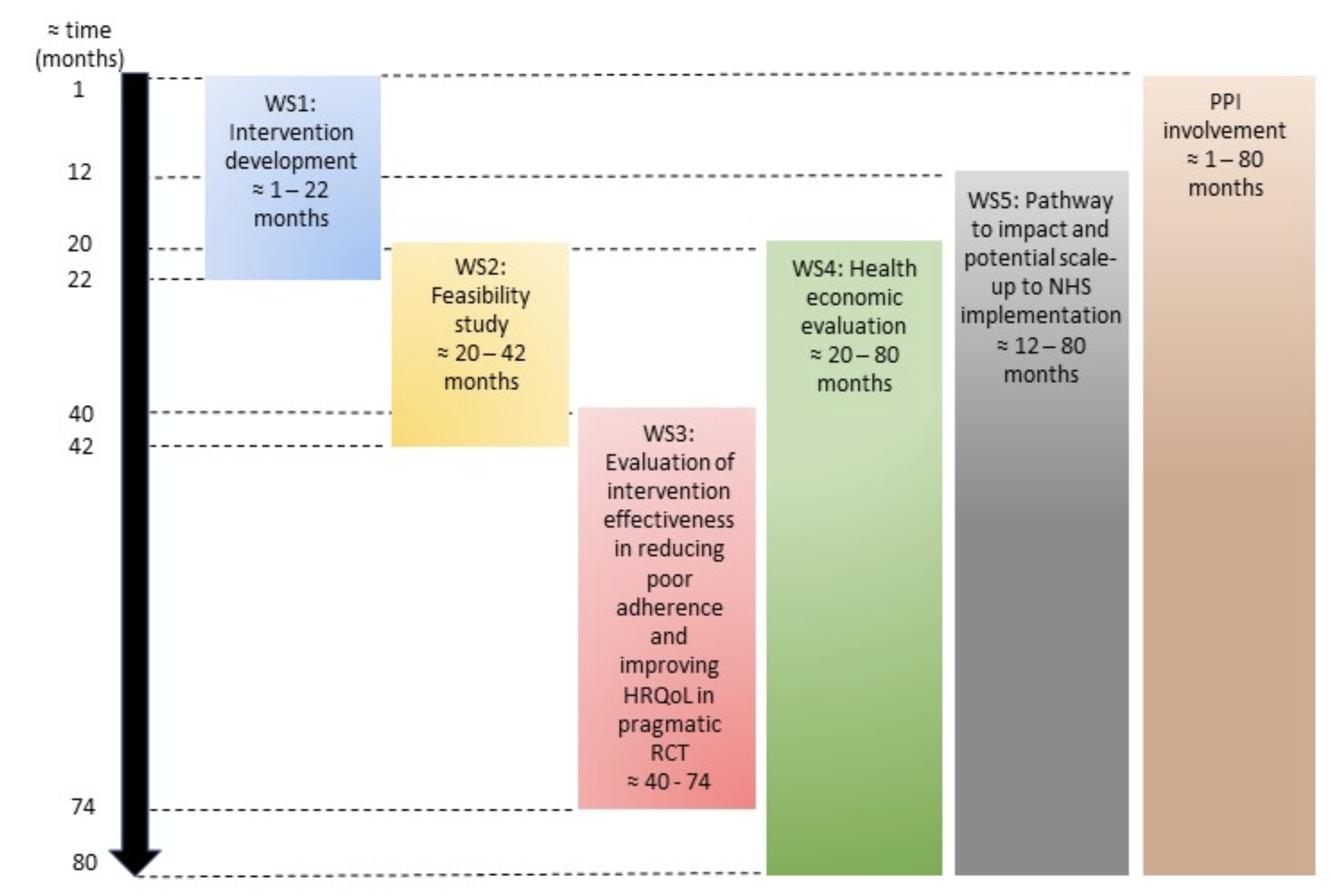
Workstream 1 involved the design, development and optimisation of the HT&Me support package. It was developed using an evidence-based and theory-informed approach. Patient and public involvement (PPI) has been integral to all stages of design and development.
Within this workstream we conducted two optimisation studies to collect user feedback. This informed improvements to the HT&Me support package. Preliminary data suggests the HT&Me support package is acceptable and engaging to users.
The diagram below provides an overview of the support package development, design and optimisation process.
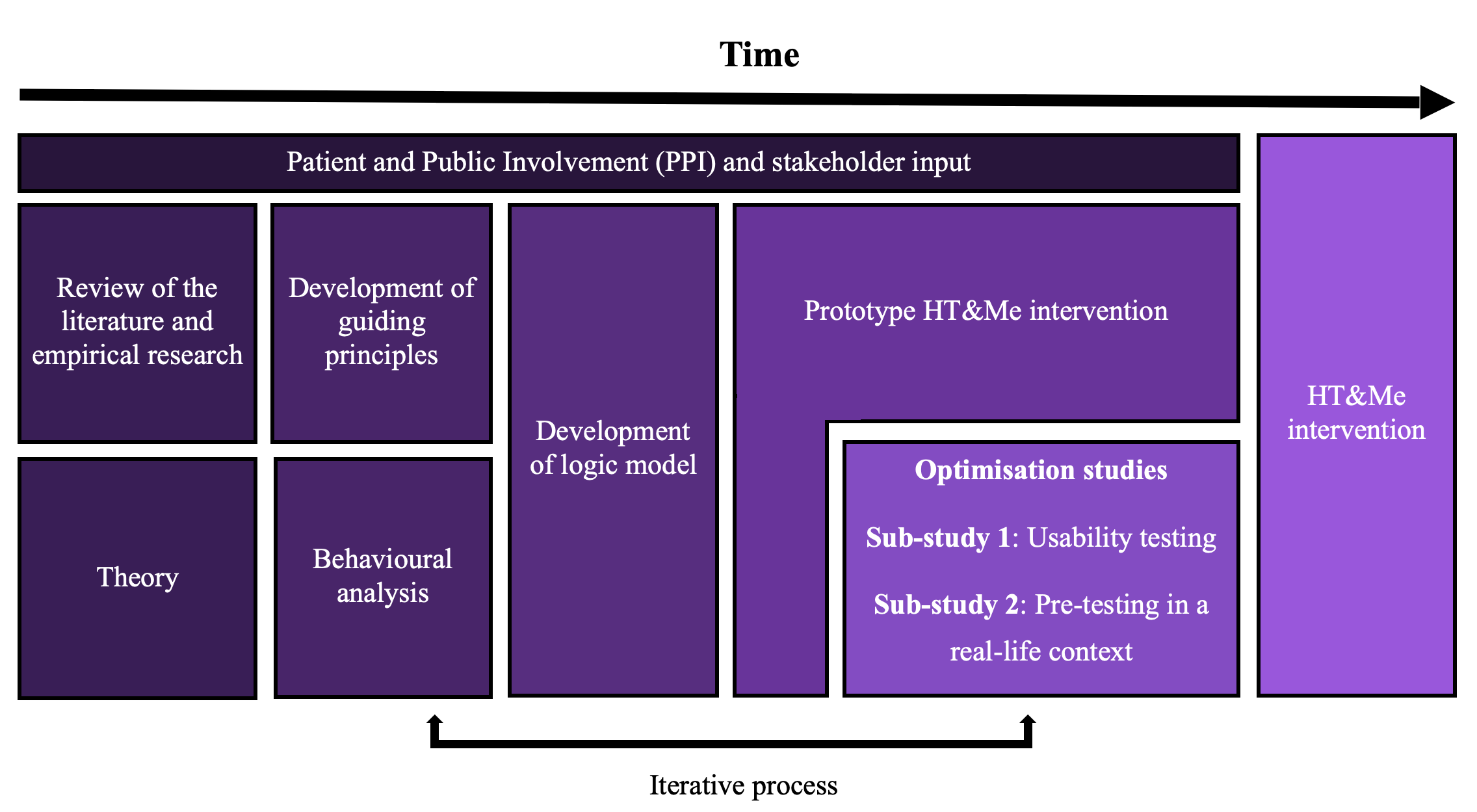
Workstream 1 is complete, and the development process has been published: Click Here to read
Workstream 2 assesses the feasibility of the HT&Me support package for both users and healthcare professionals, across two sub-studies. To do this, we have recruited 59 women who had been prescribed hormone therapy for early breast cancer across 5 NHS sites in England to our first sub-study. Women received the HT&Me support package for 8 weeks alongside their usual care and completed a questionnaire before they received the support package, and then a second one 8 weeks later. We interviewed 20 of these women to hear about their experiences of the HT&Me support package. We also interviewed 13 of the key healthcare professionals that were involved in delivering the support package across our sites to gather feedback on the study processes. Data has been useful to inform the randomised control trial (RCT) (Workstream 3).
In a second sub-study, where 56 women were recruited, we have tested the feasibility of accessing, and analysing, prescription encashment and GP prescribing data to objectively measure adherence to hormone therapy.
We have recruited women from the following sites:
- Gateshead Health NHS Foundation Trust
- Great Western Hospitals NHS Foundation Trust
- Imperial College Hospital Trust
- Newcastle-upon-Tyne Hospitals NHS Foundation Trust
- Oxford University Hospitals NHS Foundation Trust
Feasibility study documentation
Please note these are for the Feasibility study and therefore are NOT currently in use for the RCT.
- To access the participant information sheet for the SWEET Feasibility study, CLICK HERE
- To access the participant consent forms (face-to-face and verbal) for the SWEET Feasibiility study, CLICK HERE
The SWEET Feasibility data controller is Newcastle upon Tyne Hospitals NHS Foundation Trust (NuTH)
Privacy notice: Click here
Email for Data Protection Officer at NuTH: nuth.dpo@nhs.net
The SWEET Feasibility data processor is Newcastle University
Data protection policy at Newcastle University: Click here
For more info on how we use your information, Click here
For a link to the information commissioner’s office, Click here
Email for patient relations (NuTH): patient.relations@nuth.nhs.uk
Workstream 3 involves the delivery of a randomised control trial (RCT) alongside a process evaluation.
We are investigating whether the HT&Me support package improves adherence to hormone therapy and cancer-specific health-related quality of life. To do this, a total of 1460 women with early breast cancer will be randomised to receive i) either the HT&Me support package alongside their usual care, or ii) usual care alone. Women will be asked to complete questionnaires when they sign up to the study (baseline), and then 6, 12 and 18-months later. These will measure their adherence to hormone therapy, quality of life, and other additional factors that might impact the effectiveness of the HT&Me support package.
Recruitment opened in March 2024, and will take place over two years.
If you are a healthcare professional at an NHS site that delivers breast cancer care and would be interested in finding out more about your site taking part, please click here.
If you are interested in taking part as a participant, please contact your hospital to see if they are taking part in the SWEET study, and whether you are eligible to take part.
Participant information sheet for Workstream 3 (RCT)
You can access the current version of the participant information sheet for workstream 3 as a PDF file here.
Workstream 4 includes: i) analyses to establish how cost-effective the support package is within the context of the randomised control trial (RCT) (Workstream 3), ii) a budget impact analysis, and iii) modelling of long-term cost-effectiveness for the recommended 5-10+ year period of hormone therapy treatment.
Cost-effectiveness is likely to be influenced by variables including the extent to which the support package increases adherence to hormone therapy, patient age, disease stage, and adherence. The impact of these with other variables will be explored in sensitivity and subgroup analyses.
Workstream 5 seeks to inform the potential implementation of HT&Me within the NHS. We will be using findings from our discussions and interviews with healthcare professionals conducted during Workstreams 1, 2 and 3, to guide this. In particular, we will identify the factors that are most important to delivery and how we can maximise the potential impact for patients, and others involved throughout the programme.
To scale up our impact beyond the research programme, we will i) form close working relationships with charities, the NHS and commissioning bodies, ii) link with the NHS Long Term Plan, and iii) engage with the NIHR Digital Strategy.
- More information to follow
- Another project
